What is Matter? Science for Kids
Recently we explored What is an Element? While learning about Elements, we heard about Matter. Although we briefly touched on matter, we thought this was an important subject, one we should dig into a little deeper. So today let’s explore: What is Matter?
What is Matter in Science?
Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases.
Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
The Definition of Matter
As a quick definition, matter is any physical substance or the stuff things are made of. Matter has mass and volume, which means it needs to take up space.
What is Matter? Matter is anything that has mass and takes up space. It can be defined as the physical substance that makes up the universe and everything in it, including objects, liquids, gases, and plasma. All matter consists of atoms and molecules.
Matter is made of atoms
Everything around you that you can touch is matter. Atoms are the building blocks of matter. A combination of atoms forms a molecule. Large groups of atoms and molecules form the bulk matter of day-to-day life in the physical world.
An atom is part of an element and molecules. So what is the difference between an element and a molecule? A specific element, like Hydrogen (H) is composed of only one type of atom, while a molecule can have only one type of atom or different types of atoms, like water (H2O). We learned about elements in our article, What is an Element?
Although there are some different ways of defining matter, as a simplified definition we can say matter is made of atoms and anything that is made up of atoms and particles that have both mass and volume. If it takes up space, it is matter!
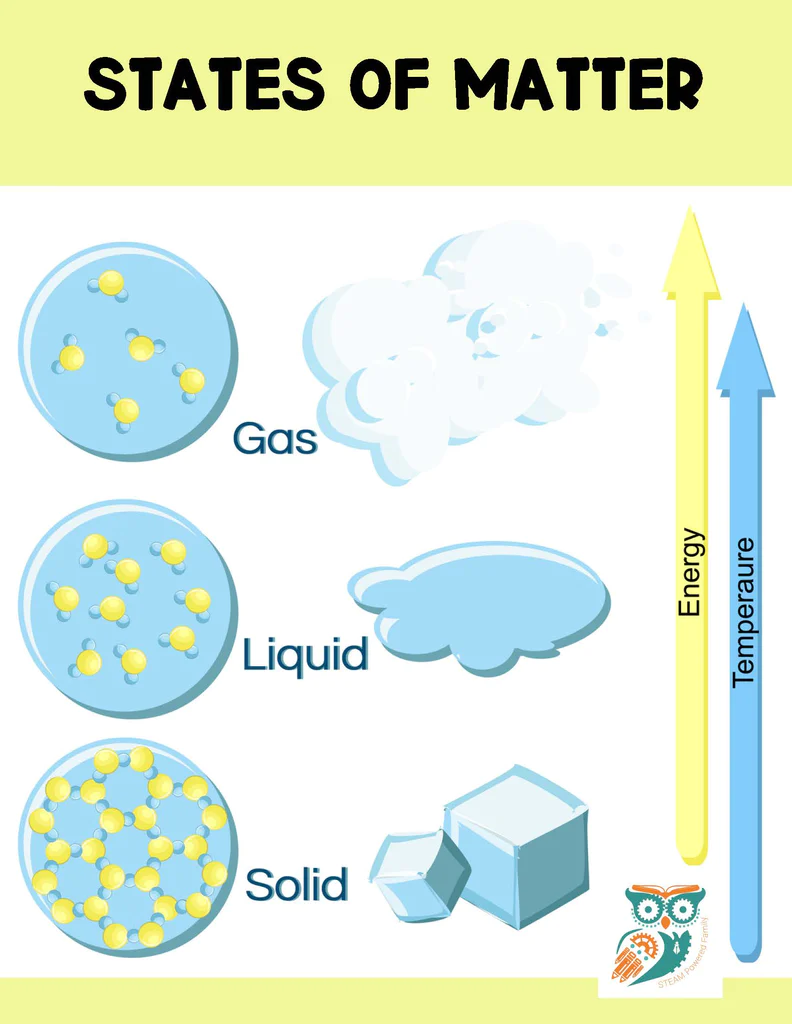
How Many States of Matter Are There?
There are four main states (also called phases) of matter, but there are more less common states of matter and even some theoretical states of matter being studied by scientists.
Matter exists in various states, the most common ones that students work with are solid, liquid, and gas. As an example, one of our favourite things to use in science experiments is water which can exist as solid ice, liquid water, and gaseous steam. Another common state of matter is plasma.
But other states are possible, including Bose–Einstein Condensates, Fermionic Condensates, and Quark–Gluon Plasma. Plus even more that scientists continue to discover and study!
The state or phase that a type of matter exists in, is affected by temperature. At room temperature, something like steel is solid, while water is a liquid. Increase the temperature and the steel start to liquify, while the water turns to gas. When matter is changed from one state to another, such as when a liquid is transformed to a gas or a solid is converted to a liquid, a change of state occurs.
Let’s dig into the different types of matter in more detail.
What is a Solid?
A solid is a state of matter in which the particles are closely packed together and vibrating in place. This results in a definite shape and volume (think of an ice cube). Unlike liquids and gases, solids have a fixed shape and volume and do not flow or expand to fill their container. Solids have a high density and pressure compared to liquids and gases. The behavior of solids is determined by the strong attractive forces between particles which hold them in place. This causes solids to be strong and rigid, making them useful for a variety of applications, such as building materials and machinery parts. Examples of solids include rock, metal, and ice.
What is a Liquid?
A liquid is a state of matter in which the particles are close together but not rigidly fixed in place like in a solid. This results in a definite volume but not a fixed shape. Unlike solids, liquids take the shape of their container (think of filling a glass with water) but have a definite volume. They have an intermediate density and pressure, right between what you would find with solids and gases. The behavior of liquids is determined by intermolecular forces, which are stronger than in gases but weaker than in solids. This gives liquids a fluidity and the ability to flow, making them useful for a variety of applications, such as lubrication and cooling. Examples of liquids include water, oil, and alcohol.
What is a Gas?
A gas is a state of matter in which the particles are widely spaced and in constant motion, resulting in the absence of a fixed shape or volume. Gases can spread out to fill any container they are placed in (picture steam from a boiling pot). They have low density and pressure compared to solids and liquids. Gases are found naturally in the Earth’s atmosphere (such as water vapour and oxygen) and can also be produced in the laboratory through various processes such as heating a solid or a liquid. Examples of gases include air, oxygen, nitrogen, and carbon dioxide.
The Fourth State of Matter – Plasma
Plasma is a state of matter in which a gas has been heated to such a high temperature that some or all of its electrons have been stripped away from their parent atoms or molecules, leaving behind a soup of positively charged ions and free electrons. Plasmas are naturally found in stars and lightning. Plasma can also be created in the laboratory. They are often referred to as the fourth state of matter, along with solids, liquids, and gases, although students in school are usually only taught the first 3 until they reach more advanced studies. Plasmas are characterized by their high electrical conductivity and their ability to generate and respond to electric and magnetic fields. Because of their unique properties, plasmas have a wide range of practical applications, including in fusion energy, plasma cutting, and plasma displays.
Now we have explored the 4 main types of matter, let’s explore matter in a hands on way!
States of Matter Example with Water
To explore states of matter when it comes to solids, liquids and gases, the best thing to use is water. Think about water in those various states, it is still water. Whether it is a glass of water you are drinking, the ice cubes in your drink, or the steam when you boil the water to make some tea. It is still water.
A water molecule is made up of 2 Hydrogen and 1 Oxygen atom, H2O. That chemical formula does not change as the water changes from solid, to liquid, to gas. It is still H2O unless a chemical reaction occurs. What does change is the amount of energy in the different states due to the changes in temperature.
One fun experiment with states of matter and water is our Simple Melting Snow experiment.
What State of Matter is Oobleck?
We love playing with Oobleck when exploring states of matter. Why? Because it breaks all the rules! Oobleck is a non-Newtonian fluid that exhibits properties of both a solid and a liquid. When a force is applied to it slowly it behaves like a liquid, but when the force is applied quickly it acts like a solid. Oobleck is simple mixture of a starch and water (check out our Oobleck Science Lab comparing different Oobleck recipes) and is an example of a viscoelastic material. It does not fit neatly into any one state of matter, as it exhibits both solid and liquid behaviors. Making and playing with Oobleck is a great addition to your studies into States of Matter. You can find all of our fun Oobleck activities here.
States of Matter Printables
We have a few different printables to supplement your studies into States of Matter. You can find these in the STEAM Powered Family Shop, simply click on the images to learn more about each of these printables.