Melting Magic ~ Snow Ice Simple Science
Snow Ice Simple Science is an experiment all ages can do and teaches valuable lessons about the molecular structure of water in ice form versus snowflake.
We have a lot of snow right now and if you are in the same boat as me, it’s a great time to do a little simple science activity that generates some great understanding about chemistry and molecular structure. Check out our Melting Magic ~ Snow and Ice Simple Science.
Simple Snow Science Experiment

Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases.
Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
It’s been snowing. They promised it would melt and go away. I’m not ready for winter to start yet, but sadly that hasn’t happened. Not only has it stuck around, but more fell!
Here in Canada we often get snow 8 months out of the year. It stinks most of the time, but it does offer up some great science activities.
Crush a bottle with your mind… and science!
So this week, to celebrate the kick off to winter, we did some snow and ice simple science.
Winter Science
Supplies
For this activity you will need 3 matching jars with lids. We used mason jars. Other than that you just need water in various states! Liquid, ice cubes and snow.
Don’t have snow? Try finding some highly crushed ice, or even better the “snow” created by a snow cone maker. Plus you get to have snow cones, win-win!
Directions
Fill the first jar with water and put on the lid. This is your control. We are working on developing our understanding of the scientific method and scientific process, and using controls and controlling variables is part of that process.
We talked about why we needed water in liquid form as our control, and it took a moment but then the boys realized that all our jars would eventually become filled with liquid water. Then we discussed the need for lids. They quickly answered that it was to prevent evaporation… and the cat playing in the water.
Very true on both accounts!
The next jar we filled with ice cubes. Use cubes, not shaved ice. You want there to be lots of air around the ice cubes. Secure the lid.
Finally, we went outside into the cold and scooped up snow and I had the kids pack the third jar full of snow. I encouraged them to pack as much snow as they possibly could into the jar. Secure the lid.

Now set the jars in a safe place to see what happens as they melt.
Integrating the Scientific Method
I asked my kids which jar they thought would end up with the most water and they both agreed it was the snow jar. They hypothesized that there was too much air in the ice cube jar.
Then we waited.

Soon things started to change.

But then something strange started happening. First, our snow melted much faster than our ice cubes, but it also resulted in very little water!

We waited for the ice cubes to finish melting which took many hours.
The results shocked the kids!
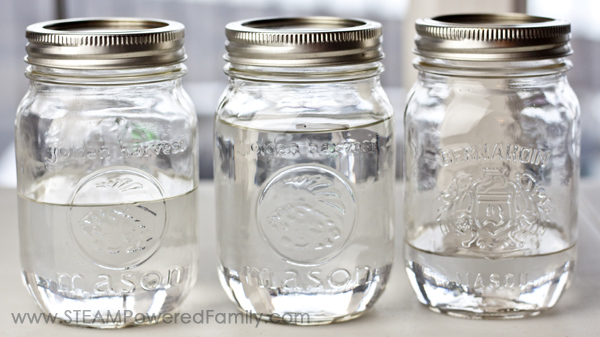
Scientific Method Printable
Join the STEAM Powered Family mailing list and unlock a scientific method printable you can use with this experiment, plus many others.
Results (and the science!)
So how did our ice cubes that left so much air in the jar result in more water than our snow? It has to do with the structure of our water molecules. Ice is the solid form of water and when in solid form the water molecules are stacked together tightly. Snow on the other hand is like ice, but it’s actually precipitation that freezes into a crystallized form. The molecules bond together into the beautiful crystal patterns we associate with snowflakes. But there is a lot of air around each crystal snowflake. Even though the kids thought they packed our snowflakes tightly, they couldn’t pack them as tightly as the molecules in our ice cubes. Hence the different results.
Happy experimenting!





